Distribution Methods Distribution procedure configuration need to permit for the continuous circulation of water within the piping by way of recirculation. Use of nonrecirculating, useless-close, or just one-way techniques or program segments really should be prevented whenever doable. If not possible, these programs should be periodically flushed and more intently monitored. Experience has proven that continuously recirculated programs are less complicated to maintain.
ENDOTOXIN CONSIDERATIONS Endotoxins are lipopolysaccharides present in and drop from the cell envelope that is exterior into the mobile wall of Gram-negative micro organism. Gram-negative microbes that variety biofilms can become a supply of endotoxins in pharmaceutical waters. Endotoxins may possibly manifest as clusters of lipopolysaccharide molecules linked to living microorganisms, fragments of lifeless microorganisms or even the polysaccharide slime bordering biofilm germs, or as no cost molecules.
Water might also remain stagnant in valves, especially at consumer details—and particularly people who not in Repeated and typical use. This can be counteracted by hygienic or “zero useless leg” valves which, Though considerably better than the alternatives (say ball valves).
Should the method is intended to become steam sanitized, very careful sloping and small-point drainage is critical to condensate removal and sanitization accomplishment. If drainage of elements or distribution traces is meant for a microbial control method, they also needs to be configured to be wholly dried applying dry compressed air (or nitrogen if ideal worker security actions are used). Drained but nonetheless moist surfaces will however guidance microbial proliferation. Water exiting from the distribution method should not be returned on the system devoid of initial passing by all or perhaps a part of the purification teach.
We remain updated with the newest regulatory adjustments, and our workforce of industry experts makes sure that your Firm remains read more compliant throughout the disposal course of action. Our extensive documentation and reporting method gives you all the required documents to display your compliance to regulatory authorities.
The amounts of DBPs generated vary with the extent and type of disinfectant used and also the concentrations and types of natural and organic supplies present in the water, which might range seasonally.
Industrial use represents an important component of the demand from customers, claims Nik Krpan, president of Cheme Engineering, a Canadian consultancy focused on water techniques for the bio/pharma industry.
Designed to satisfy the wants and budgets of expanding businesses keen on building new goods
Control of the chemical purity of those waters is significant and it is the most crucial function of the monographs With this compendium. As opposed to other official content articles, the majority water monographs (Purified Water and Water for Injection) also limit how the article can be created because of the perception that the character and robustness from the purification procedure is straight connected with the resulting purity.
Supplementing the validation upkeep method, which incorporates a system to regulate improvements to your water system and scheduled preventive maintenance, such check here as instrument recalibration.
Water-for-injection (WFI) is outlined via the US Pharmacopeia as water purified by distillation or perhaps a purification system which is equivalent or superior to distillation inside the removing of substances and microorganisms.
“The original wastewater treatment method plant at our facility in Karlskoga, Sweden was built in 2000, and this modern financial investment is usually to increase the capacity on the plant for each current and future wants.
While filtration operates effectively in principle, it is pretty high-priced for high throughputs because they will need typical altering to circumvent blockage and “increase-through.” This is why, making use of 0.22 μm filters to manage contamination in water used for merchandise manufacture is frowned on. Filters ought to be used only just before the distribution system.
There's also other types of water for which there isn't any monographs. They are all bulk waters, with names supplied for descriptive purposes only. Many of these waters are used in distinct analytical methods. The linked textual content may well not specify or indicate sure quality characteristics or modes of preparation. These nonmonographed waters may not necessarily adhere strictly towards the stated or implied modes of preparation or characteristics.
 Molly Ringwald Then & Now!
Molly Ringwald Then & Now!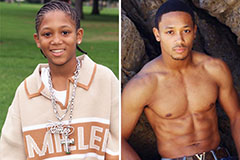 Romeo Miller Then & Now!
Romeo Miller Then & Now! Tahj Mowry Then & Now!
Tahj Mowry Then & Now! Macaulay Culkin Then & Now!
Macaulay Culkin Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!